
About the Company:
Windlas Biotech Ltd is amongst the top five players in the domestic pharmaceutical formulations contract development and manufacturing organization (“CDMO”) industry in India in terms of revenue.
Company has over two decades of experience in manufacturing both solid and liquid pharmaceutical dosage forms and significant experience in providing specialized
capabilities.
In addition to providing services and products in the CDMO market, company also sells its own branded products in the trade generics and OTC markets as well as export generic products to several countries.
As of December 31, 2020, company had a network of over 850 stockists and distributors spread across 14 states in India having grown from over 400 stockists and distributors, as of March 31, 2018
Company has three distinct strategic business verticals (“SBVs”):
- CDMO Services and Products.
- Domestic Trade Generics and Over-the-counter (“OTC”) Brands.
- Exports.
CDMO Services and Products.
Company’s CDMO Services and Products SBV is focused on providing products and services across a diverse range of pharmaceutical and nutraceutical generic products for Indian and multinational pharmaceutical companies who market such products under their own brand names to the end users.
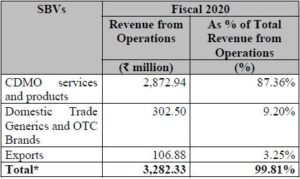
In Fiscal 2020 and the nine months ended December 31, 2020, CDMO Services and Products SBV accounted for 87.36% and 85.73%, respectively, of the total revenue from
operations.
Company has developed relationships with various leading Indian pharmaceutical companies, including Pfizer Ltd, Sanofi India, Cadila Healthcare / Zydus Healthcare , Emcure Pharmaceuticals, Eris Lifesciences, Intas Pharmaceuticals and Systopic Laboratories.
In Fiscal 2020, company provided CDMO services to seven of the top 10 Indian formulations pharmaceutical companies
Domestic Trade Generics and OTC Brands.
The Domestic Trade Generics and OTC Brands SBV consists of (i) trade generic products; and (ii) OTC brands, which include nutraceutical and health supplement products that do not require prescription and are marketed, distributed and promoted in India under company’s own brand names through online and offline channels and majorly manufactured by the company.
Company’s Domestic Trade Generics and OTC Brands SBV accounted for 9.20% and 10.12% of total revenue from operations in Fiscal 2020 and the nine months ended December 31, 2020, respectively.
Exports Business.
Company’s Exports SBV is engaged in identifying high growth markets and opportunities in semi-regulated international markets as well as selected regulated markets, for developing and registering product applications to obtain marketing authorizations for generic medicines and health supplements and subsequently, sell such products to pharmaceutical companies and pharmacies in the respective markets.
In Fiscal 2020 and the nine months ended December 31, 2020, company’s Exports SBV accounted for 3.25% and 3.34%, respectively, of the total revenue from operations.
Company’s Exports:
Company’s primary markets for export include Vietnam, Myanmar, Sri Lanka, Thailand, Philippines, Cambodia, Fiji, Trinidad & Tobago and South Africa. In the nine months ended December 31, 2020, company had exported over 47 products in these markets.
As of December 31, 2020, Company had obtained 59 product marketing authorizations across various export markets, such as, Cambodia, Ivory Coast, Philippines, Thailand, Vietnam, Myanmar and Sri Lanka.
Growth in Company’s Customers:

Segment wise Revenue from Operations:
Company’s Manufacturing Facilities:
Company currently owns and operates four manufacturing facilities located at Dehradun in Uttarakhand.
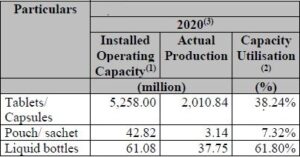
As of December 31, 2020, company’s manufacturing facilities had an aggregate installed operating capacity of 7,063.83 million tablets/ capsules, 54.46 million pouch/ sachet and 61.08 million liquid bottles.
In addition, company has also recently received a license to manufacture certain APIs at Dehradun Plant – I, which will help company with backward integration.
Company’s Capacity Utilization:
Competitors in the Business:
The domestic formulations industry is highly fragmented in terms of both, number of manufacturers and products, with 300 to 400 organised players and approximately 15,000 unorganised players.
Contract manufacturing is also characterized by high fragmentation and competition, with large number of organized and unorganized players.
The key players in domestic formulations CDMO segment include, Akums Drugs and Pharmaceuticals, Synokem Pharmaceuticals, Theon Pharmaceuticals, Innova Captab and Tirupati Medicare.
Management Team:
- Mr. Vivek Dhariwal (Designation: Chairman and Non-Executive Independent Director)
- Mr. Ashok Kumar Windlass (Designation: Wholetime Director)
- Mr. Hitesh Windlass (Designation: Managing Director)
Objectives of the Issue:
- Offer for Sale: (Rs. 236 cr)
- The Selling Shareholders will be entitled to their respective portion of the proceeds of the Offer for Sale.
Fresh Issue: (Rs.165 cr)
- Purchase of equipment required for (i) capacity expansion of existing facility at Dehradun Plant – IV; and (ii) addition of injectables dosage capability at our existing facility at Dehradun Plant-II.
- Funding incremental working capital requirements of the Company.
- Repayment/prepayment of certain of borrowings.
- General corporate purposes.
Peer Group Comparison:
- There are no listed companies in India that engage in a business similar to that of Windlas Biotech Ltd. Hence, it is not possible to provide an industry comparison in relation to the Company.
Positives for the Company:
Leading CDMO in India with a focus on the chronic therapeutic category.
- Company is amongst the top five players in the domestic pharmaceutical formulations CDMOs in terms of revenue.
- With increasing globalization and focus of large pharmaceutical players on cutting costs and optimizing operations, CDMOs have seen significant acceptance in the pharmaceutical industry internationally over the last few years.
Outsourcing Business is growing rapidly.
- With the growing demand for generic medicines and biologics, focus on reducing time to market, the capital-intensive nature of the business, and the complex manufacturing requirements, many pharmaceutical companies have identified the potential profitability in contracting with contract manufacturing and outsourcing for formulation manufacturing.
- In particular, pharmaceutical companies are increasingly outsourcing development and manufacturing of new products, and as a result, the domestic formulations CDMO market has grown at a higher rate of approximately 13% compared to the growth rate of approximately 8.6% of the domestic formulations market in the past five years.
- The increasing use of outsourcing by pharmaceutical companies for launch of new products is resulting in higher growth in the CDMO market and thereby, creating opportunities for the company.
Innovative portfolio of complex generic products supported by robust R&D capabilities.
- Company’s focus has currently been on launching new complex generic products in the chronic therapeutic category linked to lifestyle related disorders.
- Company’s complex generic products portfolio primarily comprises fixed dosage combinations, fixed dosage plus modified release combinations, customized generics and chewable or dispersible.
- The complex generic products market has a high barrier to entry as these products are generally difficult to develop and require special know-how from the development and manufacturing perspective compared to conventional generic products.
Efficient and quality compliant manufacturing facilities with significant entry barriers.
- Company currently owns and operates four manufacturing facilities located at Dehradun in Uttarakhand.
- Company’s manufacturing facilities had an aggregate installed operating capacity of 7,063.83 million tablets/ capsules, 54.46 million pouch/sachet and 61.08 million liquid bottles.
Strong and long-term relationships with leading Indian pharmaceutical companies.
- Company has developed relationships with leading Indian pharmaceutical companies, including Pfizer, Sanofi India, Cadila Healthcare / Zydus Healthcare, Emcure Pharmaceuticals, Eris Lifesciences, Intas Pharmaceuticals and Systopic Laboratories.
- Company’s operational track record in successful delivery of products, responsiveness, dosage innovation, complex generic product development, quality and technical standards, turnaround times, and productivity has facilitated the strengthening of the customer base and helped company in expanding the product and service offerings as well as geographic reach.
Company plans to Foray into high growth injectables segment.
- The margin for contract manufacturers in the injectables segment are more robust as there are fixed contracts for the development and manufacturing of the drugs and there are no selling and general costs for the contract manufacturers.
- In addition, India is a preferred outsourcing destination for injectables and has potential injectable contract manufacturing players with regulatory approved facilities capable of exporting to developed markets.
- Accordingly, company proposes to utilize Rs.50 crore of Net Proceeds towards capital expenditure for capacity expansion of the existing manufacturing facility at Dehradun Plant – IV and setting up of an injectables dosage capability at the existing facility at Dehradun Plant-II.
- Company believes that the proposed injectables business will complement the existing CDMO offerings and enable company to achieve higher margins.
Selectively pursue strategic investments and acquisitions.
- Company intends to augment organic growth by pursuing selective acquisitions and strategic alliances that will provide company with access to better infrastructure, high-value technological and operational capabilities, industry knowledge, technology expertise and geographical reach and allow company to expand the product offerings and customer base.
- Company may consider select acquisition opportunities such as acquiring divisions of existing companies to selectively expand the product portfolio, provided such opportunities offer the synergies we seek and are available at competitive prices.
Financials of the Company:
| (in Crores) | FY 18 | FY 19 | FY 20 | FY 21 |
|---|---|---|---|---|
| Revenue | 356.5 | 311.5 | 331.3 | 430.7 |
| Net Profit | 11.1 | 63.8 | 16.2 | 15.5 |
IPO Details:
| Details | Info |
|---|---|
| Issue Opens on | 4th August 2021 |
| Issue Closes on:. | 6th August 2021 |
| Issue Price | Rs.448 – 600 |
| Face Value | Rs.5 |
| Retail Category Allocation | 35% |
| Minimum Lot | 30 Shares |
| Minimum Investment | Rs. 13800 |
| Issue Constitutes | 40.05% |
| Issue Size | Rs. 401 cr ($55 million) |
| Market Cap | Rs. 1000 cr ($135 million) |
| Listing at | NSE & BSE |
| Equity Shares Offered (Fresh) | 35,86,956 |
| Equity Shares Offered (OFS) | 51,42,067 |
| Equity Shares Offered (fresh + OFS) | 87,29,023 |
| Equity Shares Prior to the Issue | 1,82,07,419 |
| Equity Shares after the Issue | 2,17,94,375 |
Also Read : List of Upcoming IPO’s in India.
Important Dates:
| Finalization of Basis of Allotment | On or Before 11th August 2021 |
| Initiation of Refunds | On or Before 12th August 2021 |
| Credit of Equity Shares: | On or Before 13th August 2021 |
| Listing Date: | On or Before 17th August 2021 |
Subscription Details: (Will be Updated)
| (Subscription-Category-Wise (no. of times) Till time : 06:00 PM) | Shares Offered | Day-1 | Day-2 | Day-3 |
|---|---|---|---|---|
| QIB | 17,23,700 | 0.00 | 0.04 | 24.40 |
| NII | 13,23,766 | 0.34 | 1.11 | 15.73 |
| Retail | 30,88,786 | 6.15 | 13.40 | 24.14 |
| Employee | ——– | —- | —– | —– |
| TOTAL | 61,36,252 | 3.17 | 7.00 | 22.40 |
IPO Valuation Parameters:
| Earnings Per Share (EPS) | Price To Earnings ratio (PE) | Return on Net Worth (RoNW) | Net Asset Value (NAV) |
|---|---|---|---|
| 8.70 | 52.87 | 18.19% | 109.36 |
| Check IPO Allotment Status: |
|---|
Link InTime : https://linkintime.co.in/PublicIssue/BSE IPO Website: https://www.bseindia.com/IPO/Allotment |
| Company Contact Info: |
|---|
| Windlas Biotech Limited 705-706, Vatika Professional Point Sector-66, Golf Course Extension Road Gurgaon 122 001 Haryana, India Tel: +91 124 2821030 E-mail: grievance@windlasbiotech.com; Website: www.windlasbiotech.com |
| IPO Registrar Info: |
|---|
| Link Intime India Private Limited C-101, 1st Floor, 247 Park Lal Bhadur Shastri Marg Vikhroli (West) Mumbai 400 083 Maharashtra, India Tel: +91 22 4918 6200 E-mail: windlas.ipo@linkintime.co.in Website: www.linkintime.co.in |



